Abstract
Cognition has a severely limited capacity: Adult humans can retain only about four items “in mind”. This limitation is fundamental to human brain function: Individual capacity is highly correlated with intelligence measures and capacity is reduced in neuropsychiatric diseases. Although human capacity limitations are well studied, their mechanisms have not been investigated at the single-neuron level. Simultaneous recordings from monkey parietal and frontal cortex revealed that visual capacity limitations occurred immediately upon stimulus encoding and in a bottom-up manner. Capacity limitations were found to reflect a dual model of working memory. The left and right halves of visual space had independent capacities and thus are discrete resources. However, within each hemifield, neural information about successfully remembered objects was reduced by adding further objects, indicating that resources are shared. Together, these results suggest visual capacity limitation is due to discrete, slot-like, resources, each containing limited pools of neural information that can be divided among objects.
Keywords: slot model, interference model, attention, hemisphere
Despite the remarkable power and flexibility of human cognition, our working memory—the “online” workspace that most cognitive mechanisms depend upon—is surprisingly limited. An average adult human has a capacity to retain only four items at a given time (1–3). This capacity is fundamental to cognition: Individual variability in capacity is highly correlated with fluid intelligence (4–6) and patients with neuropsychiatric disorders often have a reduced capacity (7, 8). Because it is so basic to cognition, capacity limitation has been well studied in humans (9), particularly the capacity limitation of visual short-term working memory (for reviews see refs. 1 and 4). This work has led to several competing theories about the neural basis of capacity limitations. “Discrete” models suggest that capacity limitations reflect a limit in the number of objects that can be simultaneously represented (3, 10, 11). “Flexible resource” models predict that only the total amount of information available is limited, with information divided among all represented objects (12–14). It is also not clear whether the limitation is in stimulus encoding or in maintenance (15).
To better understand the neural basis of capacity limitations we simultaneously recorded from single neurons in the prefrontal and parietal cortex of two monkeys trained to perform a typical human test of cognitive capacity: change localization (Fig. 1A). Two arrays of objects (colored squares) were separated by a short memory delay. In the second array, the color of a randomly chosen object (the target) was changed. Monkeys were trained to detect this change and make a saccade to it. Cognitive load was increased by varying the number of objects in the arrays from two to five. We recorded simultaneously from multiple electrodes in the frontal cortex [lateral prefrontal cortex (LPFC) and frontal eye fields (FEF)] and the parietal cortex [lateral intraparietal area (LIP)]. These areas were chosen as they are critical for short-term memory (16–19) and human studies implicate them in capacity limitations (20–22).
Fig. 1.
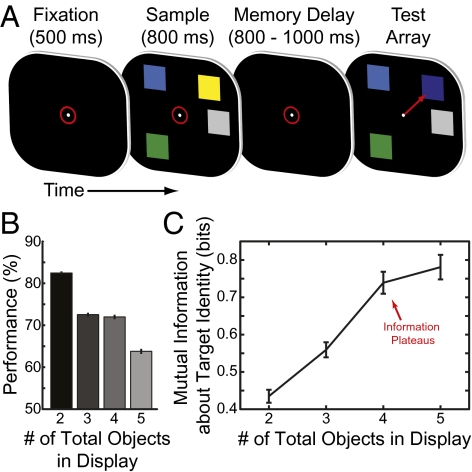
(A) Task timeline. Animals fixated (indicated by red circle) to start trial. A sample array was presented for 800 ms, consisting of two to five objects (ranging from zero to three in each hemifield). After a brief memory delay (800–1,000 ms) a test array was displayed that was identical to the sample, except one object (the target) had changed color. (B) Behavioral performance decreased as the number of objects in the stimulus array was increased. (C) The information the animal had about the entire stimulus array (derived from behavioral performance) increased until four or more objects were in the array, indicating the animal's capacity was between three and four objects. All error bars indicate 95% confidence intervals.
Results
Like in humans, increasing the number of objects decreased performance (84.8% correct with 2 objects to 66.5% with 5 objects, Fig. 1B). The amount of information the monkeys had about the objects in the array, calculated from their behavior (SI Materials and Methods), increased from 2 to 4 objects, but then saturated, reflecting a limited capacity (Fig. 1C; 2 < 3, P < 10−15; 3 < 4, P < 10−15; 4 < 5, P = 0.12, two-tailed permutation test). A capacity-limited model fitted the animals’ information significantly better than a simpler linear model (P = 0.026, validation test, see SI Materials and Methods for details). Both monkeys’ capacities were similar (3.88 monkey Sp, 3.87 monkey Si), with an overall average of 3.88 objects [95% confidence interval (CI): 3.82–3.93; SI Materials and Methods]. In similar paradigms the capacity of an average adult human is typically around 4 items (1).
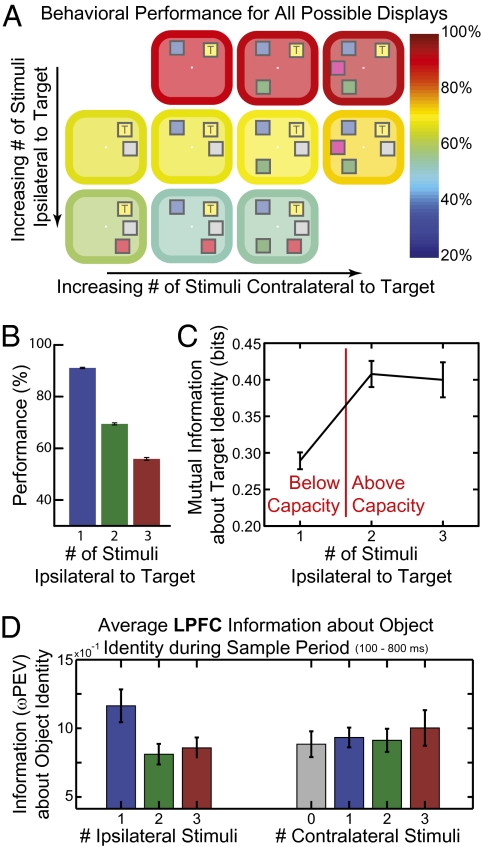
Closer examination of the monkeys’ behavior revealed that their total capacity was composed of two independent, smaller, capacities in the right and left halves of visual space (hemifields). Adding objects to the same hemifield as the target strongly degraded performance (Fig. 2A, rows), whereas adding objects in the opposite hemifield had no impact (Fig. 2A, columns; ipsilateral effect, P = 5.0 × 10−11; contralateral effect, P = 0.23; ANOVA). The number of same-hemifield objects accounted for >95% of the behavioral effect of the total number of objects. Behavioral performance was not significantly different between the two hemifields, suggesting similar capacity in each hemisphere (target hemifield effect, P = 0.74; ANOVA). The information the animals had about each hemifield increased with a second object, but then saturated, suggesting each hemifield's capacity was ∼2 objects (Fig. 2C; 2 > 1, P < 10−15; 3 > 2, P = 0.58, two-tailed permutation test). Again, a nonlinear, capacity-limited model fit significantly better than a linear one (P = 4.4 × 10−4, validation test). The estimated per-hemifield capacity was 1.6 objects (95% CI: 1.60–1.64; capacity of 1.74 for Sp and 1.51 for Si, Fig. S1).
Fig. 2.
(A) Behavioral performance (indicated by the color of the border/background) for all possible stimulus displays. Adding objects to the same side (ipsilateral) as the target (marked with a “T”) impaired performance (rows), whereas adding objects to the other side (contralateral) had no effect. This result argues for separate capacities in each hemisphere. (B) Behavioral performance decreased as the number of objects ipsilateral to the target was increased and (C) the information the animal had about the ipsilateral stimulus array (derived from behavioral performance) increased until two or more objects, reflecting the animal's capacity was between one and two objects in each hemifield. (D) Information was lost in lateral prefrontal cortex (LPFC) neurons over the sample period (100–800 ms after stimulus array onset) as items were added ipsilateral to the encoded object (Left). However, adding contralateral items (Right) had no impact on neural information, matching the observed behavioral effects. All error bars indicate 95% confidence intervals.
Neural activity also showed independent capacities for each visual hemifield. For example, average object information in LPFC neurons during the sample period decreased with increasing number of objects in the same hemifield (Fig. 2D, Left), but not the opposite hemifield (Fig. 2D, Right; ipsilateral effect, P = 1.4 × 10−4; contralateral effect, P = 0.51; two-way ANOVA). We found the same effect during the memory delay [ipsilateral effect (Ipsi), P = 2.6 × 10−6; contralateral effect (Contra), P = 0.53; two-way ANOVA] as well as in the LIP and FEF (Ipsi, P = 0.026 and P = 0.029; Contra, P = 0.52 and P = 0.19; two-way ANOVA; see SI Materials and Methods for details). This result cannot reflect attention to one hemifield or switching attention between them; the monkeys could not predict which object would change and performance was well above that expected from those strategies.
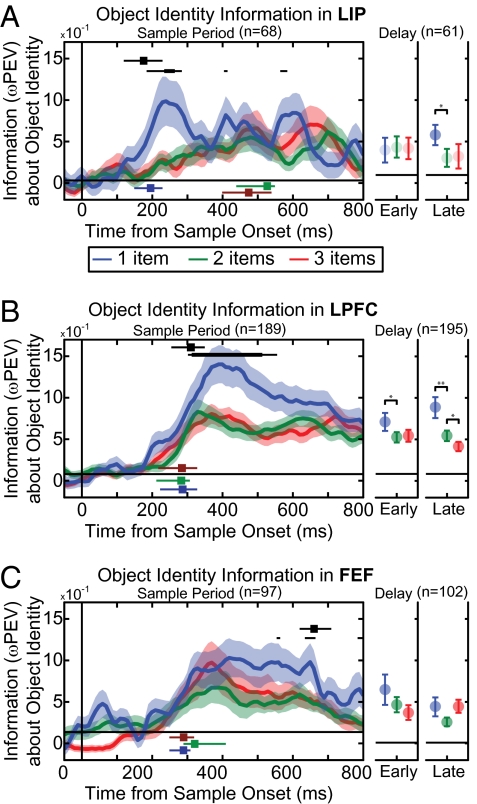
Is the brain's capacity limit a bottom–up failure to perceptually encode objects or a top–down failure to retain objects in memory? A perceptual encoding failure predicts that, when capacity is exceeded, object information is lost during neurons’ sensory responses and in lower-order before higher-order cortex. A memory failure predicts a loss later (after encoding) and in higher cortical areas first. We found the former. Below capacity (one object per hemifield), object information appeared in LIP early after sample array onset [193 ms (95% confidence interval, 149–229 ms); Fig. 3A], followed by LPFC [317 ms (249–359 ms); Fig. 3B] and FEF [291 ms (249–339 ms); Fig. 3C, LIP < the LPFC, P < 10−3; LIP < FEF, P = 0.003, randomization test], consistent with a bottom–up flow of sensory information from parietal to frontal cortex. By comparing below- to above-capacity activity, we computed the latency for information loss (Fig. 3, compare blue curve to red/green curves). Information loss began soon after array onset and immediately in LIP activity [191 ms (139–249 ms); Fig. 3A, black square], earlier than in the LPFC [341 ms (289–379 ms); Fig. 3B, black square] and FEF [658 ms (599–709 ms); Fig. 3C, black square, LIP < LPFC, P < 10−3; LIP < FEF, P < 10−3; LPFC < FEF, P < 10−3, randomization test]. In fact, when capacity was exceeded (two to three objects per hemifield), information in the LIP was weak and did not reach significance until after the LPFC and FEF (all P < 10−3, Fig. 3 A–C and SI Materials and Methods). Individual neurons showed similar effects, with >85% of neurons selective in both below- and above-capacity displays showing a decrease in information when displays were above capacity (P = 4 × 10−8 across all areas; Fig. S3 and SI Materials and Methods). Information loss above capacity carried through the memory delay (Fig. 3 A–C, Right) with the LPFC showing a further reduction of information for three vs. two objects late in the delay (P = 0.047, randomization test). One possibility is that this delayed difference between two- and three-item displays reflects an unbounded (or less limited) source of information available during sample presentation but lost during the memory delay (e.g., iconic memory, ref. 2).
Fig. 3.
Average information about object identity for object-selective neurons in (A) the lateral intraparietal cortex (LIP), (B) the lateral prefrontal cortex (LPFC), and (C) the frontal eye fields (FEF). Information about an encoded object is shown when the object was presented alone (blue), with another object (two total items, green), and with two other objects (three total objects, red). The shaded region indicates SEM. (A–C, Left) Information is shown over time for the sample period. The time at which information about the object was above baseline is indicated for one, two, and three items as blue, green, and red squares with associated lines depicting 95% CI. Information is lost in all three areas when the stimulus array is above the animal's capacity (i.e., information with two and three objects is less than with one object). Significant difference between one and two or three objects is indicated by black bars (thin bars, P < 0.05; thick bars, P < 0.01). The time to first significant loss (P < 0.05) is shown as a black square at the top of each section, with horizontal lines indicating 95% CI. We computed the neural latency for object selectivity as the time of the steepest rise in neural information around when it first became significant. This statistic is resistant to changes in statistical power, allowing for comparison across areas (SI Materials and Methods). The timing of information loss (first in LIP, followed by LPFC, and then FEF) suggests information is lost in a bottom–up manner. (A–C, Right) Information over early (first 400 ms) and late (second 400 ms) in the delay period. Dark shading of circles indicates significant information above baseline. Significant differences between one or two and two or three objects are indicated in black above.
A limited capacity to represent multiple objects was evident on a behavioral and neural level. However, what is the neural mechanism underlying this bottleneck? Two main hypotheses have been put forth. First, objects could compete for encoding within a limited number of discrete “slots”, with each object being either successfully or unsuccessfully encoded (3, 10, 11). Alternatively, capacity limitations could reflect a limited information “pool” that is flexibly divided among objects, and so adding objects reduces the information allotted to each encoded object (12–14). Our behavioral and neural data suggest that the two hemifields act like two slots. We tested whether encoding was also slot-like within a hemifield.
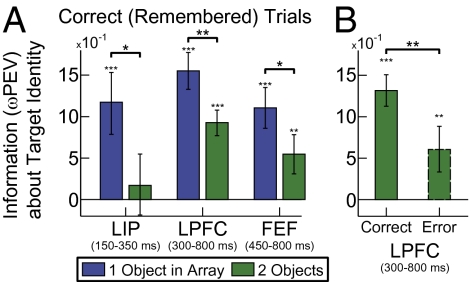
A pure slot-like model predicts that encoding an object is all or none: If successfully remembered, there should be an equal amount of information about it regardless of how many other objects are in the array. If an object is forgotten, there should be no information about it. In contrast, we found that even when a given target object was successfully encoded and retained, information about that specific object was reduced in all three areas when another object was added to its hemifield (Fig. 4A, 1 > 2 objects: LIP, P = 0.033, 150–350 ms after array onset; LPFC, P = 0.001, at 350–800 ms; FEF, P = 0.041 at 450–800 ms, permutation tests; time windows selected on the basis of periods of selectivity; Fig. 3). Further, when the change to an object was not detected, it was not completely missing from neural activity: There was still significant information in LPFC neurons during the presentation of the sample array (Fig. 4B, P = 0.0052, permutation test), albeit significantly reduced from correct trials (Fig. 4B, P = 0.008, permutation test) that continued into the memory delay (P < 0.001, permutation test).
Fig. 4.
(A) Neural information about the identity of the target is significantly lower with a second object, even when the animal correctly identified the change at the target. (B) Significant information about target identity is found even on error trials when the animal did not detect the target change, although it is significantly lower than during correct trials. All error bars indicate SEM and significance is as follows: *P < 0.05; **P < 0.01; and ***P < 0.001.
Discussion
We report three main results. First, capacity limits were seen in the initial sensory encoding and not as a memory failure. When capacity was exceeded, information was lost during the initial neural response to the stimulus and in parietal before frontal cortex. This result is supported by previous work showing that a subject's ability to attentively filter information is a major factor in their effective capacity size (15, 23). It is also consistent with our second main result of two independent capacities in the right and left half of visual space. The bottleneck begins in posterior cortex where neural receptive fields are more restricted to one hemifield than in prefrontal cortex. Human studies report varying degrees of hemifield independence (24–27). In our task, the need to localize the changed object may emphasize this independence. Indeed, the strongest evidence for human hemifield independence comes from divided-attention tasks like multiple-object tracking (28, 29).
Discrete-resource models suggest that capacity depends on a limited number of slot-like, independent resources (3, 10, 11) whereas flexible-resource models suggest a resource pool that can be subdivided among items (12–14). Our results suggest both mechanisms are at play. The two hemifields act like discrete resources, whereas within a hemifield neural information is divided among objects in a graded fashion. This model is supported by human psychophysical experiments indicating graded information resources within slots (30, 31). It is also consistent with observations that information about multiple objects multiplexes in PFC neurons as if the objects are shared among them (32, 33). One intriguing possibility is that the neural mechanism underlying the observed decay in information is similar to the competition observed during inattention (34–36), although here the animal's task is to attend to and remember all of the stimuli. The fact that contralateral stimuli had little impact on neural representations (Fig. 2D) suggests an independence at the neural level, possibly reflecting competition within contralaterally biased receptive fields (even in the lateral prefrontal cortex) (18, 37). Interestingly, parietal neurons seem to have a more severe capacity limitation than frontal regions. Indeed, there were no above-capacity responses until after activation of frontal cortex, suggesting top–down influences may be needed to partially overcome capacity limits. This finding also necessitates that information reaches prefrontal cortex from sources other than parietal. An obvious candidate is the ventral stream, suggesting further experiments are needed to fully understand the dynamics of capacity limits throughout the visual system.
In sum, our results suggest that visual capacity limits result from competition for encoding within independent, but limited pools of neural information that can each be divided among multiple objects.
Materials and Methods
Please see SI Materials and Methods for a detailed description of all materials and methods.
Animals and Recordings.
Two adult rhesus monkeys (Macaca mulatta) were trained to perform a change localization task (Fig. 1A). After a short fixation period (500 ms) an array of colored squares was presented for 800 ms (the sample period). A long sample period was chosen to ensure the animal had enough time to fully attend to and process all of the items in the array. Following the sample period the stimuli were removed for a memory delay that ranged from 800 to 1,000 ms. A second array was then presented that was identical to the sample array except the color of a single randomly chosen object (the target) was changed. The animal was rewarded for making a single, direct, saccade to it. Six new stimulus locations were chosen each day, ranging from ±75 angular degrees from the horizontal meridian and between 4° and 6° of visual angle (dva) from fixation. Stimuli were colored squares 1 dva on a side. Two colors were randomly chosen for each location every day, preventing the monkeys from adopting any long-term memorization strategies. An infrared-based eye-tracking system monitored eye position at 240 Hz (ISCAN). Behavioral control of the paradigm was done with the Monkeylogic program (www.monkeylogic.net) (38, 39).
Simultaneous recordings were made from single neurons in prefrontal cortex (LPFC, 584 neurons; FEF, 325 neurons) and the parietal cortex (LIP, 284 neurons). All procedures followed the guidelines of the Massachusetts Institute of Technology Committee on Animal Care and the National Institutes of Health. Epoxy-coated tungsten electrodes (FHC) were used for recording. Electrodes were lowered using a custom-built microdrive assembly with 1-mm spacing.
Estimating Behavior Capacity.
The animal's behavioral capacity was estimated using an information theoretic approach. This method fully accounts for chance behavior and makes no assumption about the animal's strategy in solving the task. First, the animal's information about each display was determined from its behavioral performance (see SI Materials and Methods for details). By combining these we could determine the information the animal had about displays with a given total number of objects (Fig. 1C). Hemifield information was determined by decomposing the total information in a given array into each hemifield's display (SI Materials and Methods). Again, this information can be combined for displays of a given size to estimate the animal's capacity in each hemifield (Fig. 2C).
Neural Information About Stimulus Identity.
We quantified the information each neuron's firing rate carried about the identity (color) of each object in the hemifield contralateral to the recorded hemisphere using a bias-corrected percentage of explained variance (ωPEV) statistic (Fig. S2 and SI Materials and Methods). To ensure our analysis was unbiased, all well-isolated neurons were recorded and our analysis made no a priori assumptions about the structure of color or location information in neural activity across time or display conditions. We report all neurons that showed significant information (i.e., object selectivity) to any stimulus in the sample array (68 neurons in LIP, 189 neurons in LPFC, and 97 neurons in FEF). Information was averaged across all selective neurons and all attempted trials, unless specified otherwise (such as for the correct or error-only trials, Fig. 4).
Timing of Information Loss.
Two different latencies were of interest: When did neurons first encode information about a stimulus and when was this information degraded due to capacity limitations? For the former, we asked when the amount of information in a neuron population significantly exceeded baseline. For the latter, we determined the latency of a significant difference in neural information between below- and above-capacity conditions (e.g., one vs. two objects). For both measures we defined the latency as the time point of maximum rise in the difference function. The maximum rise statistic was used as it is resistant to differences in statistical power: Varying the number of neurons in a population will change the threshold of significance, but will not a priori affect the shape of the function and therefore will not change the point of maximum slope. The search for the point of maximum rise was limited to a 150-ms window around the first time a significant difference was found (e.g., 191 ms in LIP for greater information in below-capacity trials compared with above-capacity trials). Uncertainty about the time to significance was determined by bootstrapping the population of neurons and redetermining the point of maximum slope.
Supplementary Material
Acknowledgments
We thank S. Henrickson and M. Wicherski for comments on the manuscript; W. Asaad, K. Maccully, and D. Ouellette for technical and other support; and V. Yorgan, R. Meyer, and S. Greenman for assistance with training. E.K.M. and co-workers are supported by National Science Foundation CELEST OMA-0835976 and National Institute of Mental Health Grant 1R01MH091174-01.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1104666108/-/DCSupplemental.
References
- 1.Cowan N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behav Brain Sci. 2001;24:87–114. doi: 10.1017/s0140525x01003922. discussion 114–185. [DOI] [PubMed] [Google Scholar]
- 2.Sperling G. The information available in brief visual presentations. Psychol Monogr. 1960;74:1–29. [Google Scholar]
- 3.Luck SJ, Vogel EK. The capacity of visual working memory for features and conjunctions. Nature. 1997;390:279–281. doi: 10.1038/36846. [DOI] [PubMed] [Google Scholar]
- 4.Fukuda K, Vogel E, Mayr U, Awh E. Quantity, not quality: The relationship between fluid intelligence and working memory capacity. Psychon Bull Rev. 2010;17:673–679. doi: 10.3758/17.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cowan N, et al. On the capacity of attention: Its estimation and its role in working memory and cognitive aptitudes. Cognit Psychol. 2005;51:42–100. doi: 10.1016/j.cogpsych.2004.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engle RW, Tuholski SW, Laughlin JE, Conway AR. Working memory, short-term memory, and general fluid intelligence: A latent-variable approach. J Exp Psychol Gen. 1999;128:309–331. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- 7.Lee EY, et al. Visual working memory deficits in patients with Parkinson's disease are due to both reduced storage capacity and impaired ability to filter out irrelevant information. Brain. 2010;133:2677–2689. doi: 10.1093/brain/awq197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karatekin C, Asarnow RF. Working memory in childhood-onset schizophrenia and attention-deficit/hyperactivity disorder. Psychiatry Res. 1998;80:165–176. doi: 10.1016/s0165-1781(98)00061-4. [DOI] [PubMed] [Google Scholar]
- 9.Marois R, Ivanoff J. Capacity limits of information processing in the brain. Trends Cogn Sci. 2005;9:296–305. doi: 10.1016/j.tics.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Awh E, Barton B, Vogel EK. Visual working memory represents a fixed number of items regardless of complexity. Psychol Sci. 2007;18:622–628. doi: 10.1111/j.1467-9280.2007.01949.x. [DOI] [PubMed] [Google Scholar]
- 11.Fukuda K, Awh E, Vogel EK. Discrete capacity limits in visual working memory. Curr Opin Neurobiol. 2010;20:177–182. doi: 10.1016/j.conb.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilken P, Ma WJ. A detection theory account of change detection. J Vis. 2004;4:1120–1135. doi: 10.1167/4.12.11. [DOI] [PubMed] [Google Scholar]
- 13.Bays PM, Husain M. Dynamic shifts of limited working memory resources in human vision. Science. 2008;321:851–854. doi: 10.1126/science.1158023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvarez GA, Cavanagh P. The capacity of visual short-term memory is set both by visual information load and by number of objects. Psychol Sci. 2004;15:106–111. doi: 10.1111/j.0963-7214.2004.01502006.x. [DOI] [PubMed] [Google Scholar]
- 15.Vogel EK, McCollough AW, Machizawa MG. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005;438:500–503. doi: 10.1038/nature04171. [DOI] [PubMed] [Google Scholar]
- 16.Xu Y, Chun MM. Dissociable neural mechanisms supporting visual short-term memory for objects. Nature. 2006;440:91–95. doi: 10.1038/nature04262. [DOI] [PubMed] [Google Scholar]
- 17.Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science. 1971;173:652–654. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- 18.Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- 19.Curtis CE, D'Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci. 2003;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- 20.Todd JJ, Marois R. Posterior parietal cortex activity predicts individual differences in visual short-term memory capacity. Cogn Affect Behav Neurosci. 2005;5:144–155. doi: 10.3758/cabn.5.2.144. [DOI] [PubMed] [Google Scholar]
- 21.Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;428:751–754. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- 22.Palva JM, Monto S, Kulashekhar S, Palva S. Neuronal synchrony reveals working memory networks and predicts individual memory capacity. Proc Natl Acad Sci USA. 2010;107:7580–7585. doi: 10.1073/pnas.0913113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428:748–751. doi: 10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- 24.Delvenne JF. The capacity of visual short-term memory within and between hemifields. Cognition. 2005;96:B79–B88. doi: 10.1016/j.cognition.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Delvenne JF, Kaddour LA, Castronovo J. An electrophysiological measure of visual short-term memory capacity within and across hemifields. Psychophysiology. 2010;48:333–336. doi: 10.1111/j.1469-8986.2010.01079.x. [DOI] [PubMed] [Google Scholar]
- 26.Voytek B, Knight RT. Prefrontal cortex and basal ganglia contributions to visual working memory. Proc Natl Acad Sci USA. 2010;107:18167–18172. doi: 10.1073/pnas.1007277107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barceló F, Suwazono S, Knight RT. Prefrontal modulation of visual processing in humans. Nat Neurosci. 2000;3:399–403. doi: 10.1038/73975. [DOI] [PubMed] [Google Scholar]
- 28.Alvarez GA, Cavanagh P. Independent resources for attentional tracking in the left and right visual hemifields. Psychol Sci. 2005;16:637–643. doi: 10.1111/j.1467-9280.2005.01587.x. [DOI] [PubMed] [Google Scholar]
- 29.Cavanagh P, Alvarez GA. Tracking multiple targets with multifocal attention. Trends Cogn Sci. 2005;9:349–354. doi: 10.1016/j.tics.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Zhang W, Luck SJ. Discrete fixed-resolution representations in visual working memory. Nature. 2008;453:233–235. doi: 10.1038/nature06860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson DE, Vogel EK, Awh E. Precision in visual working memory reaches a stable plateau when individual item limits are exceeded. J Neurosci. 2011;31:1128–1138. doi: 10.1523/JNEUROSCI.4125-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Ninokura Y, Mushiake H, Tanji J. Representation of the temporal order of visual objects in the primate lateral prefrontal cortex. J Neurophysiol. 2003;89:2868–2873. doi: 10.1152/jn.00647.2002. [DOI] [PubMed] [Google Scholar]
- 33.Warden MR, Miller EK. The representation of multiple objects in prefrontal neuronal delay activity. Cereb Cortex. 2007;17(Suppl 1):i41–i50. doi: 10.1093/cercor/bhm070. [DOI] [PubMed] [Google Scholar]
- 34.Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- 35.Heeger DJ. Normalization of cell responses in cat striate cortex. Vis Neurosci. 1992;9:181–197. doi: 10.1017/s0952523800009640. [DOI] [PubMed] [Google Scholar]
- 36.Reynolds JH, Heeger DJ. The normalization model of attention. Neuron. 2009;61:168–185. doi: 10.1016/j.neuron.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rainer G, Asaad WF, Miller EK. Memory fields of neurons in the primate prefrontal cortex. Proc Natl Acad Sci USA. 1998;95:15008–15013. doi: 10.1073/pnas.95.25.15008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asaad WF, Eskandar EN. A flexible software tool for temporally-precise behavioral control in Matlab. J Neurosci Methods. 2008;174:245–258. doi: 10.1016/j.jneumeth.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asaad WF, Eskandar EN. Achieving behavioral control with millisecond resolution in a high-level programming environment. J Neurosci Methods. 2008;173:235–240. doi: 10.1016/j.jneumeth.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.