Enzymes are amazing catalysts. These proteins are made of nothing more than a handful of Earth-abundant elements, and they promote a vast array of reactions, convert chemical energy to physical motion, and act with remarkable specificity. In many cases, we have struggled to find non-enzymatic catalysts that can drive some of the same chemical reactions.
Unfortunately, there isn't an enzyme for many reactions we would sorely like to catalyze—things like digesting plastics or incorporating carbon dioxide into more complex molecules. We've had a few successes using directed evolution to create useful variations of existing enzymes, but efforts to broaden the scope of what enzymes can do have been limited.
With the advent of AI-driven protein design, however, we can now potentially design things that are unlike anything found in nature. A new paper today describes a success in making a brand-new enzyme with the potential to digest plastics. But it also shows how even a simple enzyme may have an extremely complex mechanism—and one that's hard to tackle, even with the latest AI tools.
Ending esters
The reaction the research team worked on (involving some of the same people who designed snake venom inhibitors) is the breakdown of what's called an ester bond. Ester bonds are formed by linking two chains of carbon atoms by an oxygen atom, with one of the flanking carbons being linked to a second oxygen. These can be broken apart by adding a water molecule, which leaves one carbon chain linked to an alcohol (COH) group and the other an organic acid (COOH).
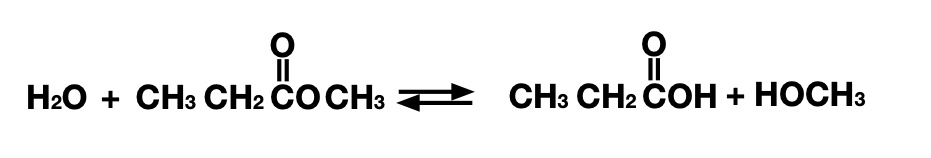
These bonds show up in various biomolecules, so there are many enzymes that can manipulate them. But beyond biology, they also show up in a number of plastic polymers that we use on a large scale—polyester got its name due to how many instances of the chemical bond show up in it. So there's a lot of potential value in being able to break down ester bonds. And we have plenty of examples from biology to show us how it's done.

 Loading comments...
Loading comments...
